Medical Steel Door
Safe and durable: Made of 304 stainless steel or high-quality galvanized steel plate, impact-resistant and corrosion-resistant, with a service life of >100,000 times.
Clean and antibacterial: Seamless surface treatment, easy to clean and disinfect, optional antibacterial coating, effectively reducing the risk of hospital infection.
Sealed sound insulation: Multiple sealing design of door seams, sound insulation >30dB, ensuring a quiet and sterile medical environment.
Efficient fire protection: Meets Class A fire protection standards (fire resistance ≥1.5 hours), flame retardant and heat insulation enhances safety.
Intelligent adaptation: Supports electric opening and closing, card swiping linkage, and automatic door closers to improve the efficiency of medical processes.
Medical steel door product description
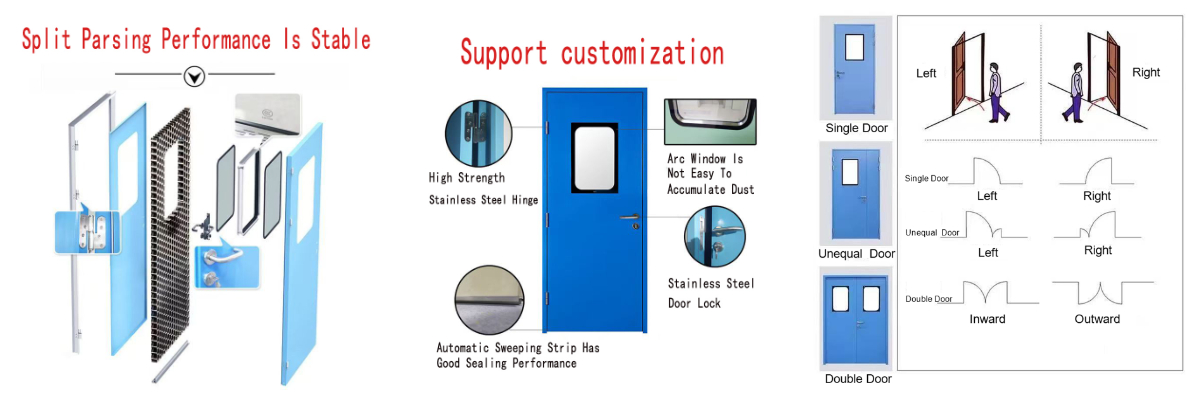
Structure and process
The door body base material is 1.2mm thick 304 stainless steel or galvanized electrolytic steel plate (tensile strength ≥500MPa), and the interior is filled with 120kg/m³ high-density fireproof rock wool (fire-resistant temperature ≥1100℃). The door body is integrated through laser seamless welding technology, and the surface is covered with nano-level antibacterial powder coating (in accordance with ISO 22196 standard, antibacterial rate >99.9%), and the overall thickness is precisely controlled at 50mm±2mm. The seamless design completely eliminates sanitary dead corners and can withstand high-frequency scrubbing of highly corrosive disinfectants such as sodium hypochlorite every day.
Double safety certification protection
Fireproof performance: passed GB 12955-2015 Class A fireproof certification, fire resistance limit ≥1.5 hours, back-fire surface temperature rise <140℃;
Impact resistance: static compressive strength of door body ≥800N, hinge load-bearing capacity of 200kg (3 times higher than the national standard), can withstand high-speed impact of hospital beds and medical equipment without deformation.
Medical-grade dynamic sealing system
The door frame integrates a three-layer sealing structure: EPDM airtight strips to achieve basic sealing, magnetic adsorption strips to enhance closing tightness, and the bottom automatic lifting baffle (sealing force>40N) forms physical isolation at the moment of closing the door;
Sound insulation performance>28dB (in line with GB/T 8485 standard), airtightness reaches ISO Class 8 clean level (air leakage ≤0.02m³/h·m).
Intelligent medical ecological integration
Supports non-contact induction opening and closing (sensing distance 0-30cm adjustable), hospital access control system linkage, and automatic release of safe passages when power is off;
Hydraulic door closer opening and closing speed is adjustable in 4 gears (90° opening takes 3-8 seconds), and automatically rebounds within 0.3 seconds to prevent pinching when encountering obstacles.
Key technical parameters
Door opening adaptation range: width 800-2000mm, height 2100-2400mm (according to GB/T 5824-2021 standard, support non-standard customization);
Surface physical properties: coating hardness ≥ 2H (pencil hardness test GB/T 6739-2006), scratch resistance is 3 times higher than ordinary medical doors;
Mechanical durability: passed 150,000 fatigue opening and closing tests (QB/T 3893-1999), equivalent to 10 years of high-frequency use in hospitals;
Radiation protection options: observation window lead equivalent 0.5-2.0mmpb (meet GBZ 130-2020 radiation protection requirements).
Special adaptation for medical scenarios
Operating room/ICU: airtight structure maintains positive pressure environment and blocks the transmission chain of microorganisms;
Negative pressure isolation ward: dynamic sealing system stably maintains ≥5Pa pressure difference (in line with WHO isolation standards) to prevent the spread of pathogens;
Biological laboratory/hazardous chemical pharmacy: explosion-proof multi-point locks + acid and alkali resistant coating, adapted to strong corrosion environment;
Public medical channel: 1.2mm thickened stainless steel anti-collision guard plate, to resist 200+ impacts of bed pushers per day.
Certification and service system
The product has passed the YY/T 0627-2023 medical door industry standard and EU CE-MDR medical device directive certification, and provides triple test reports for fire resistance, air tightness, and radiation protection. The main structure has a 10-year warranty, supports BIM modeling pre-installation and smart hospital system integration, and provides a full life cycle safety management solution for medical buildings.